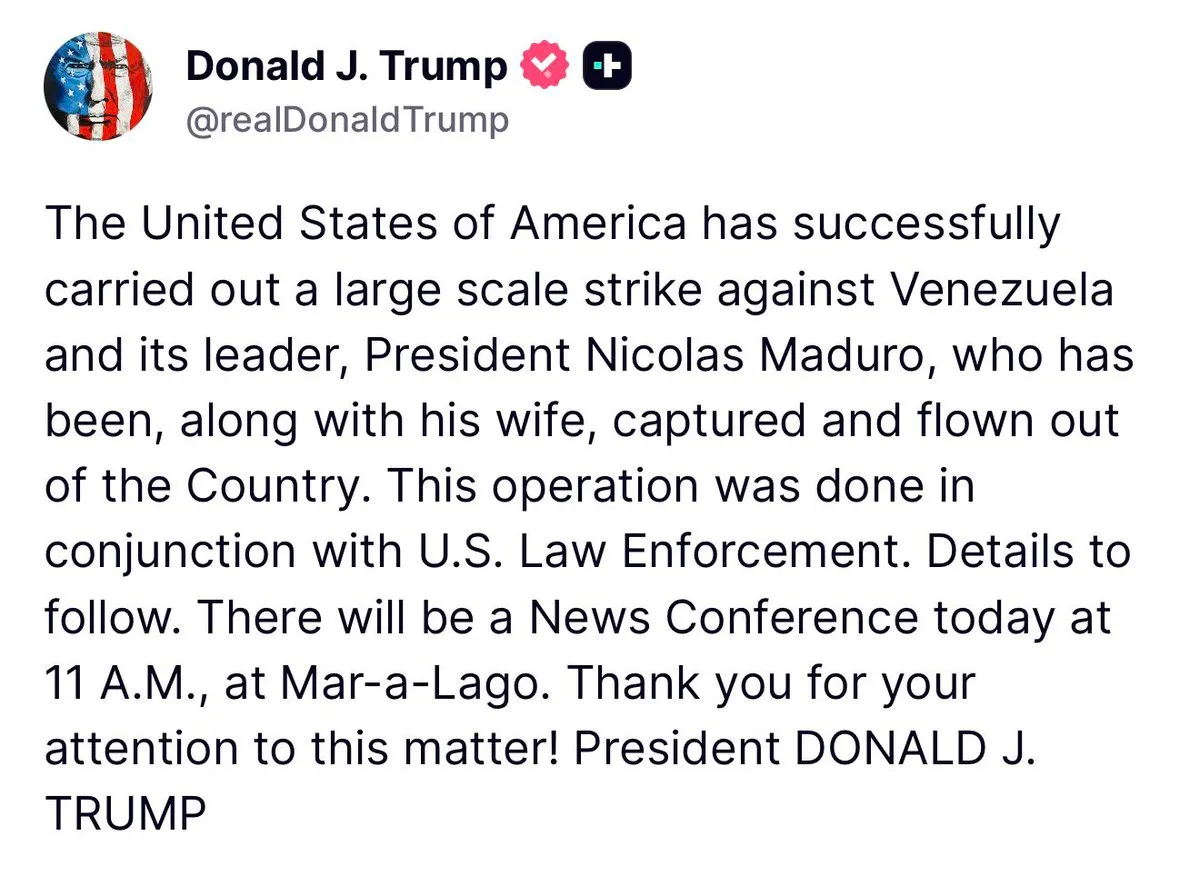
U.S. Captures Venezuelan President Nicolas Maduro and wife

FBI Disrupts Alleged New Year’s Eve Attack, Man Charged with Attempting to Provide Material Support to ISIS

‘Tim Walz and the Fraudsters Aren’t Escaping’: Influencer Exposes Potential $110 Million Minnesota Child-Care Scam

Justice Department Announces Seizure of Stolen-Password Database Used in Bank Account Takeover Fraud
Just In Time For Christmas, Nation Gifts Service Members $1,776 'Warrior Dividend'
Recalls
Meijer is announcing a voluntary recall of select Meijer branded Greek and Low-fat yogurt as a precautionary measure due to the potential risk of a small glass particles. Meijer became aware of the issue when a customer returned a yogurt…
Weis Markets has issued a recall for its store-made Weis Quality Dried Beef Party Rye dip for failing to list egg and milk allergens on its label. This product was sold in eleven Weis Markets’ stores including most of its…
Smokehouse Pet Products, Inc. of Sun Valley, CA is recalling 4-oz bags of dog treats labeled as “Beefy Munchies,†because it has the potential to be contaminated with Salmonella. Salmonella can affect animals eating the products and there is risk…
Raws for Paws of Minneapolis, MN is recalling approximately 4,000 pounds of its 5 lb. and 1 lb. chubs of Ground Turkey Pet Food because they have the potential to be contaminated with Salmonella. Salmonella can affect animals eating the…
Emergency Medicine, Risk Manager, Nursing ISSUE: Philips is recalling the HeartStart MRx Defibrillator due to a defect in the device's Gas Discharge Tube (GDT). The GDT has micro cracks which allows internal gasses to escape and causes the tubes to…
BETHESDA, MD. – BrainScope Company, Inc., the medical neurotechnology company which created the first FDA-cleared handheld medical device, BrainScope One, for assessment of the full spectrum of traumatic brain injury, announced it will immediately commence development of a pediatric capability.…
The U.S. Food and Drug Administration has permitted marketing of the Philips IntelliSite Pathology Solution (PIPS, Philips Medical Systems Nederland B.V.), as an aid to the pathologist to review and interpret digital images of surgical pathology slides prepared from formalin-fixed…
The U.S. Food and Drug Administration has approved Odactra, the first allergen extract to be administered under the tongue (sublingually) to treat house dust mite (HDM)-induced nasal inflammation (allergic rhinitis), with or without eye inflammation (conjunctivitis), in people 18 through…
The U.S. Food and Drug Administration has approved Vonvendi, von Willebrand factor (Recombinant), for use in adults 18 years of age and older who have von Willebrand disease (VWD). Vonvendi is the first FDA-approved recombinant von Willebrand factor, and is…
The U.S. Food and Drug Administration today approved the first generic version of Nexium (esomeprazole magnesium delayed-release capsules) to treat gastroesophageal reflux disease (GERD) in adults and children ages 1 and older. Esomeprazole is a proton pump inhibitor that reduces…
LV8702V is optimized to reduce motor noise, vibration and heat generation, and deliver no-load power consumption savings of up to 80 percent *1 High-Efficiency Stepper Motor Driver PHOENIX, Ariz. ON Semiconductor Nasdaq: ONNN),driving innovation in energy efficiency, has introduced the…
Newest FS Series 3 Module is Latest Milestone on Cost-Efficiency Roadmap TEMPE, Ariz.--(BUSINESS WIRE)-- First Solar, Inc. (Nasdaq: FSLR) has released of its most advanced thin-film photovoltaic (PV) module, the Series 3 FS-392, which is rated at 92.5 watts. The…
